Network clinical specialists
Patient Temperature Management
The Surgical Company PTM is a leading European company acting on a global level in the field of hypothermia and offering solutions for patient warming. Preventing inadvertent perioperative hypothermia helps to reduce surgical site infections, the length of hospital stays and the costs per patient.
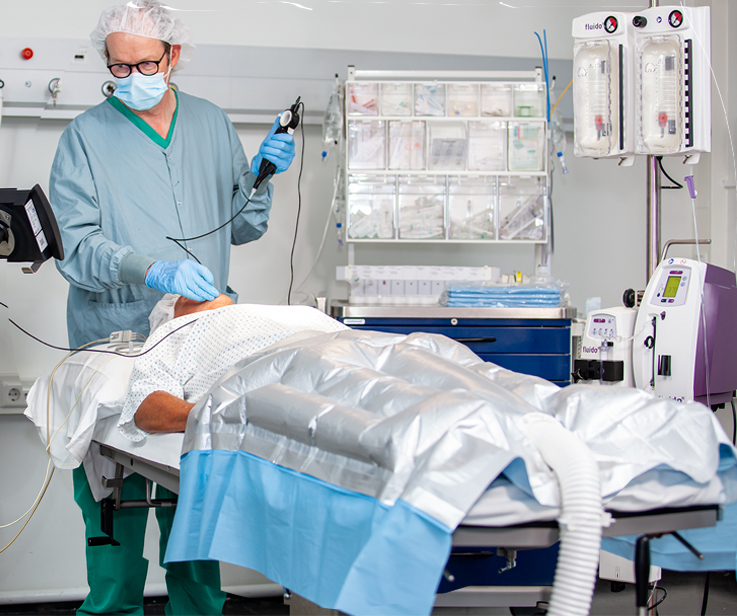
Best-in-class products

Virtual37®
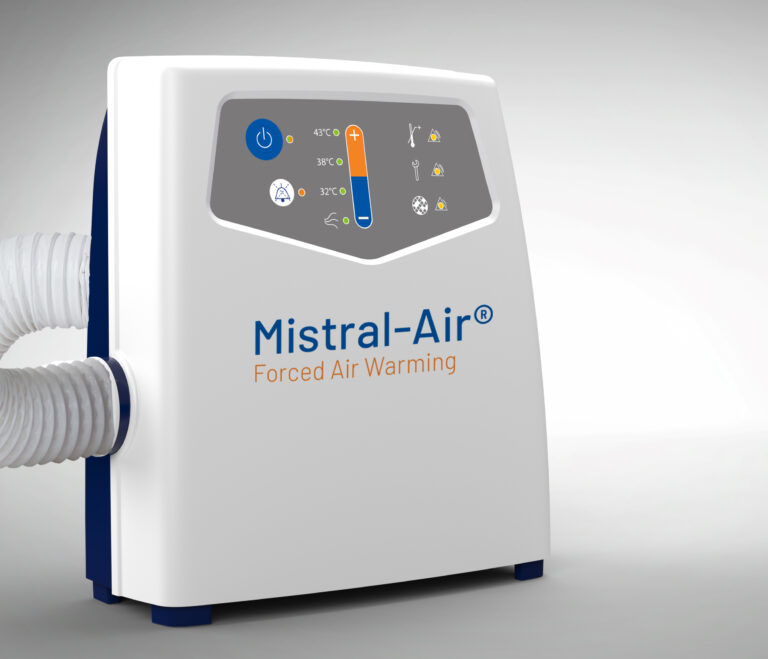
Mistral-Air® MA1200
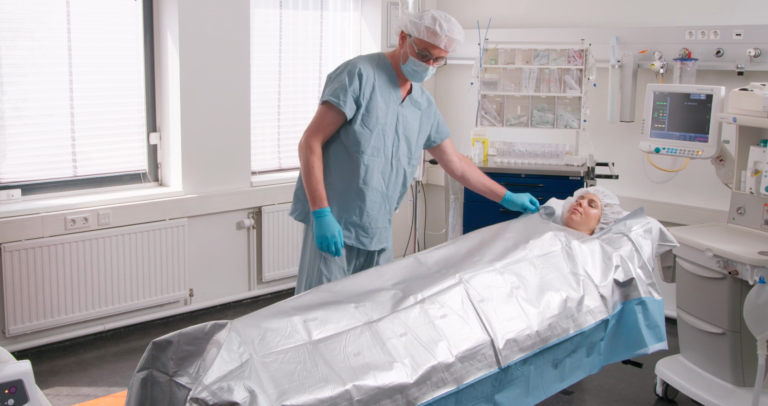
Premium Adult MA3320
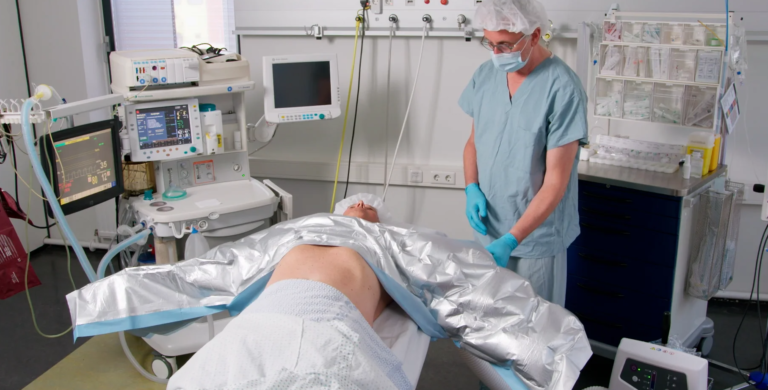
Premium Upper Body MA3360
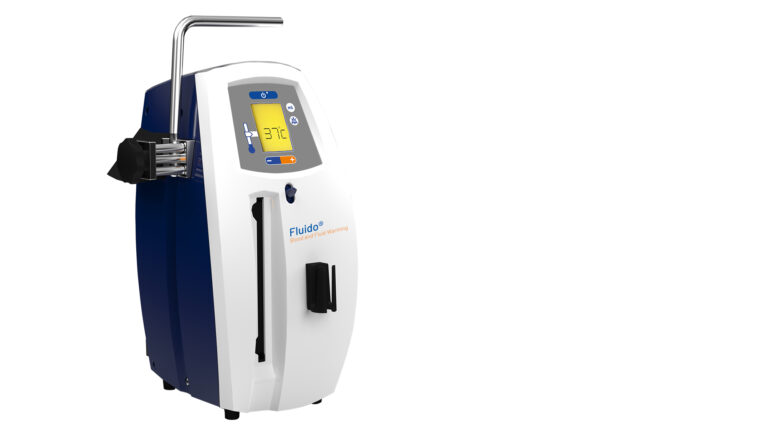
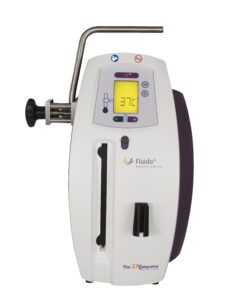
Fluido® AirGuard System

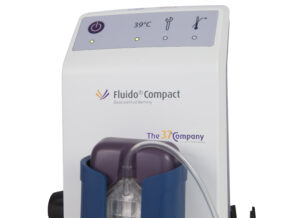
Fluido® Compact
Find your nearest PTM consultant

Our solutions for patient warming
Patient Temperature Management
Forced Air Warming
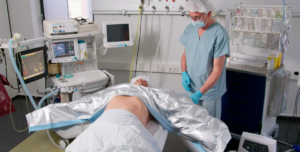
Low Flow Blood and Fluid Warming
Irrigation Fluid Warming

Heat Reflective Technology
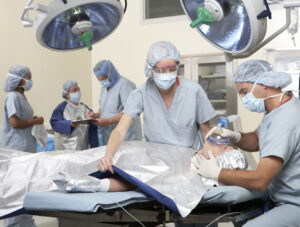
High Flow Blood and Fluid Warming
Specialization through innovation
The Surgical Company Group
60 countries
The Surgical Company PTM has become the preferred partner of hospitals and health professionals worldwide.
5 product lines
The PTM portfolio includes complete and complimentary product lines for preventing inadvertent perioperative hypothermia.
50 million patients
The Surgical Company PTM supports milions of patients worldwide maintaining normothermic, through a global network of qualified Business Partners.
3 TSC companies
The Surgical Company PTM is part of The Surgical Company Group, with sister companies Endovision and Connected Care.
How can we help you?
Video
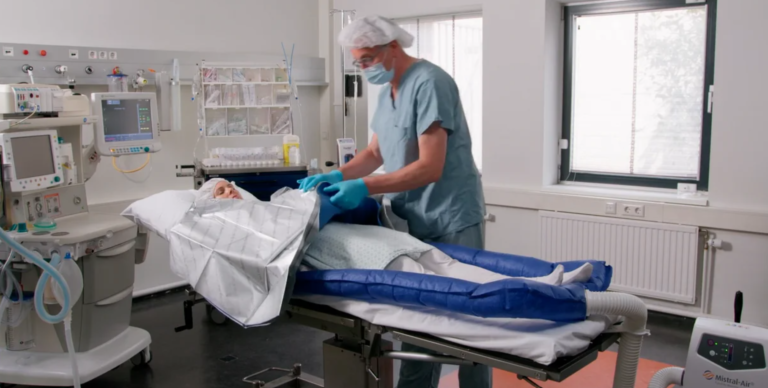
Tube MA0510
The Mistral-Air® Tube MA0510 is designed to warm your patient’s entire body in various surgical positions and can be used throughout the perioperative pathway.
The Mistral-Air® Warming System should only be operated after reading the official user ma...
Brochure
Mistral-Air® portfolio
Our complete Forced Air Warming portfolio helps healthcare professionals to prevent inadvertent perioperative hypothermia and improve patient outcome. The portfolio consists of the Mistral-Air® Forced Air Warming unit, Mistral-Air® Quick Connector, Mistral-...






